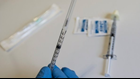
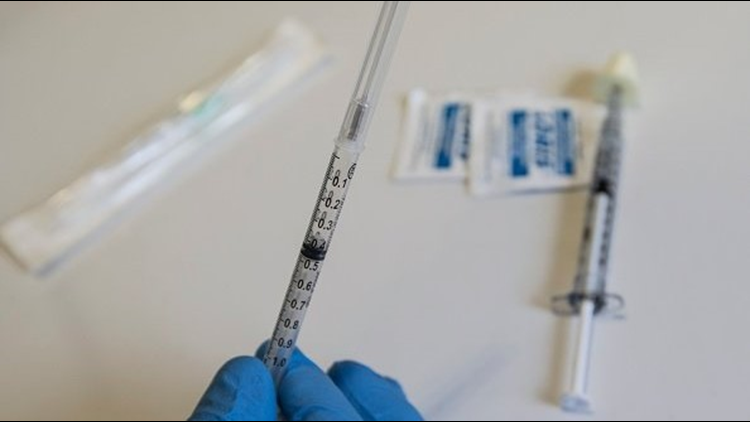
The FDA and drug maker Pfizer have issued a recall on two lots of Naloxone, the opioid overdose reversal drug, due to potential particulate matter in the syringes.
According to the FDA, Hospira Inc. issued the voluntary recall Monday. The recall includes lots 72680LL and 76510LL of Naloxone Hydrochloride Injection dated "1DEC2018" and "1APR2019" respectively. The medication is packaged in single-use cartridge syringes.
The FDA says Hospira has not received any reports of adverse events related to the products involved.
Hospira has notified retailers, hospitals and distributors of the recall to arrange for returns and exchanges on any impacted product.
Naloxone is a medication used to block the effects of opioids, often during an overdose.
Call Pfizer Safety at 1-800-438-1985 for more information.